A.2-56/2015-NIB
NATIONAL INSTITUTE OF BIOLOGICALS
(Ministry of Health & Family Welfare, Govt. of India)
A-32, Sector – 62, Institutional Area, Phase-II,
NOIDA – 201309, U.P
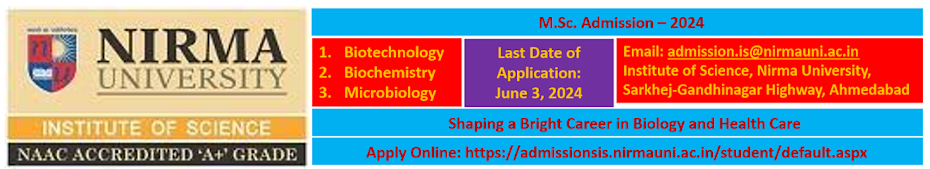
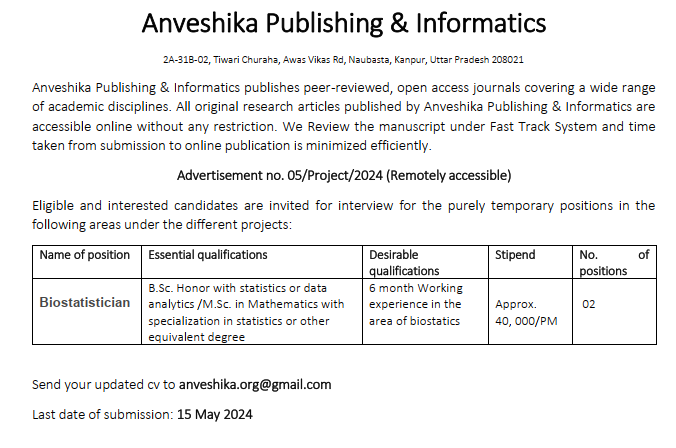
The National Institute of Biologicals, an autonomous Institute under the Ministry of Health & Family Welfare, Government of India, is a premier Scientific Organization and a Centre of Excellence to ensure quality of vaccines and biologicals in the country. Applications are invited for the following posts on direct recruitment basis:
1 Scientist Grade-II
Rs.15,600- 39,100+7600(GP)
45 yrs.
2 Scientist Grade III
Rs.15,600- 39,100+5400(GP)
35 yrs
3 Jr. Scientist
Rs.9,300-34,800 +4600(GP)
30 yrs.
The qualification(s)/experience for various posts are as under:-
1. Scientist Grade II
A. Medical (Essential) i)PG Medical Degree in the field of Microbiology / Biochemistry / Immunohaemotogoly & Blood Transfusion / Pharmacology / Bacteriology / Serology / Hematology from the recognized University and ii) Five years’ relevant experience after acquiring the essential qualification B. Non Medical (Essential) i) M. Sc in Microbiology / Clinical Microbiology / Biotechnology/ Bioinformatics/ Biochemistry / Bacteriology / Physiology/ Pharmacology/Serology/Molecular Biology from any recognized University with at least 60% marks and ii)A minimum of 7 (Seven) years’ of relevant experience in any of the following areas: a) Quality Control Testing of Biologicals and Biotherapeutics; b) Review of Technical Dossiers of Biologicals and Biotherapeutics including Vaccines in respect of Chemistry Manufacturing Control (CMC), non-clinical trial data on Animal Pharmacology/Animal Toxicology, Clinical Trial data; c) Review of Technical Dossiers of ‘In Vitro Diagnostic (IVD) Medical Devices’ in respect of conformation to the Essential Principles of Safety and Performance of Medical Devices which include Product Description, Design and Manufacturing information, Product performance specifications and associated validation and verification studies, Labelling and Regulatory history; d) Evaluation & Review of Adverse Events: i) following Immunization (AEFI) ii) associated with Biologicals and Biotherapeutics (Pharmacovigilance) iii)associated with blood transfusion and blood products administration (Haemovigilance); iv)due to indirect harm associated with IVD Medical Devices (Materiovigilance) e) Management of Quality System with application of ISO: 17025 and ISO : 34 with Total Quality Management approach . f) Research in Biologicals and Biotherapeutics, Or i)Ph.D in any of the disciplines mentioned in B(i) above, and ii) Three years’ relevant experience after acquiring Ph.D Desirable: At least 5 (five) publications or Articles in Indexed Journals, in any of the above mentioned fields of qualification or experience.
2. Scientist Grade III
A. Medical (Essential) i)PG Medical Degree in the field of Microbiology / Biochemistry / Immunohaemotogoly & Blood Transfusion / Pharmacology / Bacteriology / Serology / Hematology from the recognized University and ii) One year experience in the field after acquiring the essential qualification B. Non Medical (Essential) i) M. Sc in Microbiology / Clinical Microbiology / Biotechnology/ Bioinformatics/ Biochemistry / Bacteriology / Physiology/ Pharmacology/Serology/Molecular Biology from any recognized University with at least 60% marks and ii)A minimum of Three years’ of relevant experience in any of the following areas: a) Quality Control Testing of Biologicals and Biotherapeutics; b) Review of Technical Dossiers of Biologicals and Biotherapeutics including Vaccines in respect of Chemistry Manufacturing Control (CMC), non-clinical trial data on Animal Pharmacology/Animal Toxicology, Clinical Trial data; c) Review of Technical Dossiers of ‘In Vitro Diagnostic (IVD) Medical Devices’ in respect of conformation to the Essential Principles of Safety and Performance of Medical Devices which include Product Description, Design and Manufacturing information, Product performance specifications and associated validation and verification studies, Labelling and Regulatory history; d) Evaluation & Review of Adverse Events: i) following Immunization (AEFI) ii) associated with Biologicals and Biotherapeutics (Pharmacovigilance) iii)associated with blood transfusion and blood products administration (Haemovigilance); iv)due to indirect harm associated with IVD Medical Devices (Materiovigilance) e) Management of Quality System with application of ISO: 17025 and ISO : 34 with Total Quality Management approach . f) Research in Biologicals and Biotherapeutics, Desirable: At least 2 (Two) publications or Articles in Indexed Journals, in any of the above mentioned fields of qualification or experience.
3.Jr. Scientist
Essential: i) M.Sc. in Microbiology / Clinical Microbiology / Biotechnology/ Bioinformatics/ Biochemistry /Bacteriology / Pharmacology/ Serology / Molecular Biology/Physiology from any recognized University with at least 60% marks. and ii) A minimum of one year’s of relevant experience in any of the following areas: a) Quality Control Testing of Biologicals and Biotherapeutics; b) Review of Technical Dossiers of Biologicals and Biotherapeutics including Vaccines in respect of Chemistry Manufacturing Control (CMC), non-clinical trial data on Animal Pharmacology/Animal Toxicology, Clinical Trial data; c) Review of Technical Dossiers of ‘In Vitro Diagnostic (IVD) Medical Devices’ in respect of conformation to the Essential Principles of Safety and Performance of Medical Devices which include Product Description, Design and Manufacturing information, Product performance specifications and associated validation and verification studies, Labelling and Regulatory history; d) Evaluation & Review of Adverse Events: i) following Immunization (AEFI) ii) associated with Biologicals and Biotherapeutics (Pharmacovigilance) iii)associated with blood transfusion and blood products administration (Haemovigilance); iv)due to indirect harm associated with IVD Medical Devices (Materiovigilance) e) Management of Quality System with application of ISO : 17025 and ISO : 34 with Total Quality Management approach . f) Research in Biologicals and Biotherapeutics Desirable: At least 1 publication or an Article in an Indexed Journal in any of the above mentioned fields of qualification or experience.
General Information:
1. The prescribed Application form is available on NIB Website http://nib.gov.in and can be downloaded on or before the closing date.
2.Application form duly completed in all respects with a passport size photograph affixed at the space indicated along with self-certified copies of certificates, testimonials, caste certificate etc.and signed by the applicant should be sent to the Administrative Officer, National Institute of Biologicals (Ministry of Health & Family Welfare), A-32, Sector-62, Institutional Area, Phase-II, Noida, U.P., 201 309. The envelope containing the application form should be superscribed with “Application for the post of “_________________” and reach the Institute on or before the closing date.
The last date for submission of complete application form is 22.01.2016
Advertisement: http://nib.gov.in/Advt%20October%202015.pdf










0 Comments